New Lab Laws, Technology
Industry experts share regulatory guidelines impacting R&D; new tech for texture and product development

Microthermics’ UHT/HTST Lab-25 Direct/Indirect Processor. This pilot plant unit provides indirect as well as direct steam injection processing for products ranging from milk to tea, to puddings and cheese sauces. Manufacturers with pilot plants must be prepared to document all activities and food safety controls.

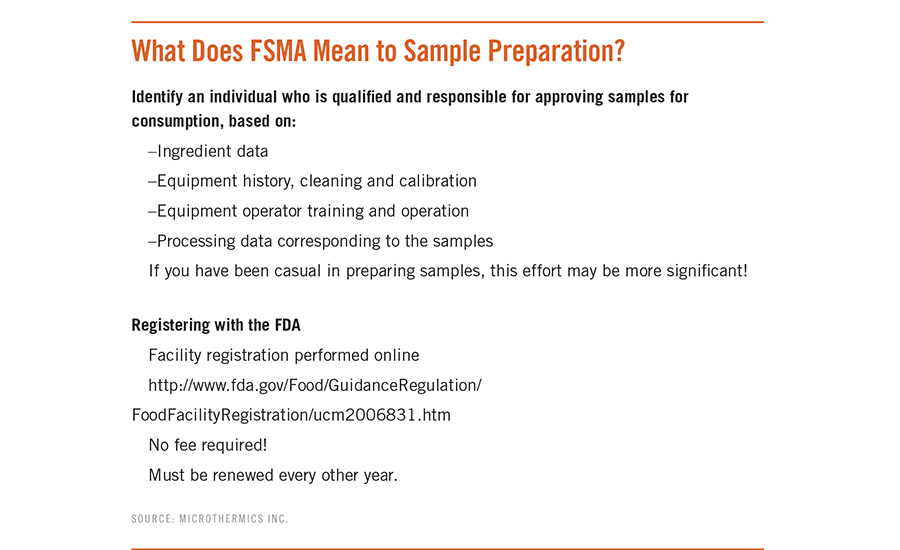
One of the requirements of FSMA is that facilities that manufacture/process, pack, or hold food used in research and development or as food samples are required to register with the FDA.

Texture analyzers offer impartial, repeatable, quick, and quantifiable results and deliver precise measurements. Now multi-plant operators have data to compare with each location and verify with a taste panel, a process that is much more under control.

New R&D software includes integrated document management; web portals for specific functionality; global regulatory compliance; enhanced people and product optimization; and better integration with business software and vendors / customers.




The Food Safety Modernization Act and How it Affects R&D
The Food Safety Modernization Act (FSMA), signed by President Obama (January 4th, 2011), gives the FDA new regulatory authority. Its ultimate goal is to provide more uniform food safety standards. However, it does leave some food formulators asking some pertinent questions, such as: What changes does FSMA entail? What does FSMA mean for R&D efforts?
At a Prepared Foods R&D Seminar presentation titled, “The Food Safety Modernization Act (FSMA) and How it Affects Research and Development,” Peter Johnson, the technical services manager for MicroThermics Inc., stated clearly that the goal of his discussion was “NOT to scare you into compliance.” Instead, he said, “We are here to share what we know about FSMA and how it can affect you.”
Johnson first broke the FSMA into its pertinent parts. The Act’s broad-sweeping authority covers almost all points of the food production chain; it applies a “preventative controls” rule; and is basically a science-based approach to food safety. The Act, says Johnson, means “FDA involvement changes from being reactive to proactive.”
When outlining the Act’s mandatory preventative controls, Johnson said the (required) preventive controls plan must include evaluating food safety hazards; specifying preventive steps or controls; specifying monitoring procedures; maintaining monitoring records; and specifying corrective actions.
Moreover, all “food facilities” are now required to register with the FDA. This includes those facilities “engaged in manufacturing/processing, packing, or holding of food for human or animal consumption in the US.” This also includes, stressed Johnson, foreign facilities for foods sold in the US.
One of the FDA website’s frequently asked questions, noted Johnson, asks if facilities that manufacture/process, pack, or hold food used in research and development or as food samples required to be registered with FDA. The FDA’s answer: “Yes.”
To further explain: “Food used in research and development or as product samples is ‘food’ for purposes of the food facility registration requirements of section 415 of the FD&C Act. Accordingly, a facility that manufactures/processes, packs, or holds food used in research and development or as product samples is required to be registered with FDA,” according to FDA.gov.
In order to prepare for FSMA, Johnson recommended that companies develop and implement a Hazard Analysis Critical Control Point (HACCP) plan. Further, it should be determined if improvements are needed (i.e., in procedures, equipment, facilities, etc.). The company should develop and use supporting documentation and also implement training and maintenance plans, Johnson advised.
There are seven HACCP steps. They are as follows:
- Conduct a hazard analysis.
- Determine critical control points.
- Establish critical limits.
- Establish monitoring procedures.
- Establish corrective actions.
- Establish verification procedures.
- Establish record-keeping procedures.
Important FSMA Implementation deadlines (as of February 2014) include:
- August 30, 2015: Preventive controls for human food.
- October 31, 2015: Produce safety, the foreign supplier verification program and third-party accreditation.
- March 31, 2016: For sanitary transport.
- May 31, 2016: Intentional adulteration.
In summary, Johnson advised, “Formalize your approach to food safety (HACCP); ensure you have the tools to implement your plan; register your facility with the FDA.” And, he stressed the importance to “keep up with this!”
“The Food Safety Modernization Act (FSMA) and How It Affects Research and Development,” Peter Johnson, technical services manager, MicroThermics Inc., pjohnson@microthermics.com, 919-878-8076, www.microthermics.com
Why Measure Food Texture?
The first thing consumers notice when they put food in their mouths is texture. Whether it is crunchy or squishy, for example, is important to the palate. And consumers are getting fussier. When they buy something repeatedly, they want it to be exactly the same—every time.
When developing a product, changes can happen when scaling up from lab to pilot plant to commercial production. These differences can be measureable using texture technology. Then the best lab product can be matched in the pilot plant—and again in the commercial run—using texture measurements. For instance, pasta texture can be tested through all stages to make sure it matches the best prototype.
“Changes in product texture can also occur when using new ingredients or when the same ingredients are not available at all production locations,” explained Al Wassilak, Midwest regional manager, Texture Technologies Corporation, in his PF R&D Seminar presentation titled “Why Measure Food Texture?”
Processing differences also affect texture. All locations may not have the exact same equipment, so the finished product may have slight differences. Texture measurements can correct for these differences. Ingredients, formula, and processing can be adjusted to meet the texture measurements needed, once the critical texture values are determined.
“For example,” Wassilak explained, “years ago, Oreo cookies were made in 15 production facilities, and each facility had their own spin on finished product. Management devoted two years to narrow these differences down and make a consistent product at all locations. It was found that everyone was making the dough a little differently. One operator added more oil to the dough to make it slide out of the blender easier. Different mix times and orders of addition of ingredients also were found. They ended up putting a texture instrument near every mixer, creating SOPs with exact formulas and orders of ingredient addition; texture measurements were taken on every batch, at specific points in the production cycle, until they were all consistent.”
Taste panels can establish likes and dislikes for texture, whether it be too crunchy or too soft, and a range that people like can be determined. But, with taste panels, everyone has different preferences, so it is not as objective a measurement as a texture measurement instrument. Many samples are needed in a test panel to get decent statistics; the panelists must be paid; and it is time consuming.
“But once the likes are established, the texture measurements can be done and statistically analyzed to give the measurements to achieve consistently,” stated Wassilak. The instrument is impartial, repeatable, quick, and quantifiable, giving very precise measurements. Now there is data to compare with each location and verify with a taste panel, a process that is much more under control.
A line of texture instruments is available with software and probes. Many features for serious research are included in the instrument and software. Tests can be automated to a certain number of cycles and replicates, and the data can be automatically posted into a spreadsheet.
“Why Measure Food Texture?” Al Wassilak, Midwest regional manager, Texture Technologies, 847-854-9536, awass@texturetechnologies.com
—Summary by Elizabeth Pelofske, Contributing Editor
The Future of Product Development Software for Food & Beverage Manufacturers
Current product development software is used for management of specifications, formulas, property and cost calculations, nutrition labeling and ingredient statements, and packaging. Most of these are stand-alone packages that provide limited collaboration with other business units. The future includes integrated document management; web portals for specific functionality; global regulatory compliance; enhanced people and product optimization; and better integration with business software and vendors / customers.
Data is an enterprise resource that must be managed from an overall enterprise perspective. Accurate data must be readily accessible by anyone who has a legitimate need. Specific data should be accessible to users in the organization, regardless of location.
Document management currently includes checking in and out, version control, audit tracking, and SharePoint integration. (SharePoint Integration enables document management capabilities in Microsoft Dynamics CRM.) The next level of document management, dubbed “intelligent reporting,” includes auto collection of common documents based on product type, including specifications, images, composition, vendors, packaging, and label information.
“Using a responsive web application which will run on any device allows collaboration for customers, salespeople, R&D, QC, and for the creation and modification of projects and briefs more conveniently,” explained Ted Pliakos, vice president of sales, Advanced Software Designs, in his PF R&D Seminar titled “The Future of Product Development Software for Food & Beverage Manufacturers.”
Scheduling timelines in the software allows management to coordinate the status of projects to make sure they stay on time and budget. Critical documents, like specifications, safety data sheets, and nutritional information, can be available for secure distribution.
Global regulatory compliance requires multi-language, country-specific nutrition facts panels, as well as global guidelines and restrictions. And nutrition labeling regulations are being updated in more than one country. Having those guidelines and restrictions in one location provides the tools necessary to maintain compliance with country, market, and/or application.
Examples Pliakos provided include sodium benzoate, which has different maximum levels permitted for EU than for Hong Kong. Crystalline silica has differing maximum levels allowed, depending on method of application. Also, restrictions on fragrances vary depending upon end-use (shampoo, cream, household cleaning, etc.) and application type (rinse-off/leave-on or non-skin contact).
“The ability to instantly determine compliance greatly reduces the risk of submitting a product for further work that may fail at a later stage,” Pliakos added.
Creating regulatory documents in multiple formats and languages gives product development the capability to automatically assess a product’s regulatory profile for any market. Software can maximize employee productivity and profits, while minimizing development and production time.
The software gives responsive formula views to assist different functional groups. The regulatory view would show the allergens and claims status of each ingredient; the nutritional view would show the nutrients provided by each ingredients; and the purchasing or inventory view would show inventory levels and vendors of each ingredients, to name some examples.
The formula optimization scenario permits developers to quickly prototype new formulas by setting targets on properties—such as percent fat, sodium, or costs—and then allowing the system to reformulate to meet those criteria.
System integration into a single integrated system means data does not have to be re-entered in multiple data bases, reducing the cost of storing and managing redundant data. Enhanced communication and collaboration across departments occurs, resulting in improved customer satisfaction.
“The Future of Product Development Software for Food & Beverage Manufacturers,” Ted Pliakos, vice president of sales, Advanced Software Designs, ted@asdsoftware.com, 636-532-6021
—Summary by Elizabeth Pelofske, Contributing Editor
Originally appeared in the February, 2016 issue of Prepared Foods as New Lab Laws, Technology.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!