Article: Cutting Sodium -- January 2008


Salt, Diet and Health
Salt in the diet is essential for healthy life, but in common with many other dietary components, too much can be harmful. A salt intake of 0.5g/day is essential to life, and a healthy person needs 1.2g/day. However, the current average daily intake of salt in the U.K. population is around 9g/day, against a target set in 1994 of 6g/day. In the U.S., dietary salt intake for men was estimated at around 10g/day in 1994, and guidelines published in 2005 recommended an upper limit of 5.75g/day. In terms of sodium consumption, the National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure recommends an upper limit of 2,400mg of sodium per day, while the average daily intake of sodium in the U.S. is 3,375mg.The main risk to health arises through increased blood pressure (hypertension) and is a consequence of the sodium content of common salt (sodium chloride). People with high blood pressure are reported to be three times more likely to develop heart disease and stroke than those with normal levels, and it is a contributory factor in 170,000 deaths a year in the U.K. It is suggested that reducing intake of salt to 6g a day would result in a 17% fall in hypertension, 6% decline in coronary heart disease and 15% fall in stroke incidence. Experts claim that these effects would be doubled or even tripled with a further reduction in salt intake. Concerns have been increasing that salt levels in the diets of children are particularly high.
A wide range of food types has been targeted for salt reduction in the U.K. Foods that have a high contribution to salt dietary intake have received particular attention, including cereals and cereal products (34% contribution) and meat, meat dishes and meat products (26% contribution). As a consequence of the high level of consumption of chilled prepared foods in the U.K., there has been considerable media coverage of excessive salt content in this category.
Functions of Salt in Food
Salt has three main functions in food: preservation against microbial growth, as a processing aid and delivery of taste and flavor. The main effect that salt has on microorganisms is to reduce the water activity of the food, so that any water within the food environment is unavailable for microorganisms to use. Salts and sugars have a similar effect, but the amount of each solute required to reduce the water activity will vary. Salt has a number of important processing and related properties. For example, salt regulates the activity of starter culture organisms, modifies enzyme activity and has a direct effect on water content on cheese during maturation. It contributes to water and fat binding in meat products and, in the bakery sector, salt has important effects on gluten development, reducing the sticky texture. It also offers a convenient vehicle for uniformly distributing micro-ingredients (flavors, vitamins, antioxidants) throughout the finished products. In any salt-reduction programs, maintaining stability against microbial growth is essential in ensuring the safety of foods. It is also important to remember that added salt is not the only source of sodium in foods, although it is generally the most important. Other sodium salts are used as preservatives (sodium benzoate), leavening agents (sodium bicarbonate), thickening agents (sodium caseinate), curing agents (sodium nitrite) and for other purposes.Salt Reduction by Stealth
Saltiness is one of the five basic tastes—the others being sweet, sour, bitter and umami (savory). The basic tastes form the foundation for food flavors, in combination with the volatile aroma components and the trigeminal components that contribute heat and cold sensations. The sensation of clean salt taste relies on the presence of both sodium ions and chloride ions, and in this lies the origin of difficulties in salt replacement. The most obvious route is to replace the sodium ion by another cation, and the most common salt replacer, potassium chloride, has seen extensive use.Potassium chloride, however, suffers from the major disadvantage that at the levels of practical interest to deliver salt taste, it also delivers a bitter taste to more sensitive palates. As sensitivity to bitterness is known to be genetically programmed, this is a problem that is difficult to overcome.
One approach that has been used effectively in many products exploits the “salami effect,” in which some product characteristic is shaved in steps that are not perceived by consumers, but which accumulate to give a substantial change. In salt-reduction programs, this assumes salt preferences are not innate, and indeed there is evidence that gradual sodium reduction over eight-12 weeks reduces liking for salty foods. This approach has been used successfully by some U.K. manufacturers in reducing salt content. For example:
Ultimately, the approach is limited by sensory, preservation and functionality issues.

David Kilcast
Saltiness Enhancement and Replacement
Salt enhancers are substances that do not have a salty taste, but enhance a salty taste when used in combination with sodium chloride. Use of these will, in principle, allow reduction in salt content, while delivering the same perceived saltiness. The scientific literature records a number of compounds that have shown some promise as enhancers, such as amino acids, lactates, trehalose (a sugar) and a byproduct of mycoprotein production that is naturally rich in ribonucleotides. The Leatherhead research showed that the amino acids’ effects were inconsistent and were often accompanied by bitter and sour off-tastes. The ingredient showed some promise in snack foods, but the most promising candidate was potassium lactate, which was effective in solution and in a range of products, with a 30% salt reduction appearing feasible.Salt replacers are substances other than sodium chloride, which do have a salty taste. Potassium chloride acts as an effective salt replacer, but at blends over 50:50 sodium chloride/potassium chloride in solution, a significant increase in bitterness and loss of saltiness is observed. Magnesium sulfate did not deliver a high level of saltiness, and in solution, was perceived as very bitter at high concentrations. The addition of magnesium sulfate to potassium chloride and sodium chloride blends in solution resulted in a decrease in saltiness and increase in bitterness, although such combinations are found in some commercial sea salts. Potassium lactate, when used to replace some of the potassium chloride in sodium chloride and potassium chloride blends in solution, reduced some of the bitterness associated with sodium chloride/potassium chloride blends.
Modification of Salt Properties
Another approach with potential in foods in which the salt is present as a coating, such as snack foods, is to increase the dissolution rate of the salt coating to deliver a more immediate saltiness “hit,” offering the possibility of reducing the overall salt content. This change in dissolution rate is thought to be a major factor in delivering the unique characteristics of some sea salts.Two approaches were taken in the project: evaluation of commercial sodium chloride forms, both as supplied and in a mixture with potassium chloride; and the development and testing of a glassy form of salt and other glassy forms produced by a freeze-drying process. Time-intensity measurements carried out by a trained sensory panel confirmed the hypothesis that smaller salt crystal sizes delivered a faster hit, and combinations of sodium chloride and potassium chloride with different particle sizes generated modified time-intensity profiles. (See chart “Saltiness and Particle Size.”) However, the relevance of this to consumer liking is not clear—do consumers want a saltiness high, or a more gradual delivery over time in the mouth?
Summary and Conclusions
The mechanisms underlying saltiness perception are deceptively simple, in comparison with the perception of other important sensory stimuli, but the lack of readily available salt substitutes limits the replacement options available to manufacturers, unlike the analogous position with sugar reduction. Additional limitations are introduced for foods in which salt has preservative and processing functions—in particular, protection against microbial growth is a prerequisite.The first step in any salt-reduction program must be to consider the viability of reduction by stealth. In the past, this has been regarded as unachievable in strongly branded products, as changes from standard quality would be unacceptable to consumers, but the success achieved by many major companies is impressive.
Salt-enhancement and -replacement options pose considerable difficulties, especially if companies wish to maintain a “clean label” policy and avoid any additional ingredients. Potassium chloride continues to show the greatest promise, but advances in the masking of the bitter note will be a prerequisite for extensive implementation. Salt reductions achieved in the canned foods sector recently have been based primarily on compensation for the salt reduction, using the addition of herbs and spices and also increasing the levels of the food components. Salt crystal habit modification is a promising method for surface-coated products.
In addition to pressures to reduce salt, there are also increasing pressures to reduce fat and sugar, and interactions must be assumed in any such programs. These interactions are likely to occur at the sensory level, but could also occur at the structural level in many products.
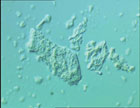
Freeze-dried Salt Microstructure
NORMAL TABLE SALT CREATED THROUGH A VACUUM-DRYING PROCESS HAS A CUBIC, CRYSTALLINE STRUCTURE AND PARTICLES IN THE 200M-500M RANGE WITH MOST 400M-500M IN SIZE. A PREMIUM, FINE, PREPARED FLOUR SALT THAT WAS VACUUM-DRIED AND GROUND HAS CUBIC, CRYSTALLINE PARTICLES GENERALLY IN THE 20M-200M RANGE WITH THE MAJORITY 50M-100M IN SIZE. AT LEATHERHEAD FOOD INTERNATIONAL, A FREEZE-DRYING PROCESS WAS USED TO CREATE A GLASSY FORM OF SALT OF VERY SMALL DIAMETER IN ORDER TO INVESTIGATE OF THE IMPACT OF SALT PARTICLE SIZE ON INTENSITY. (SEE PHOTO.) THE RESULTING SALT STRUCTURE APPEARED CUBIC, WAS THOUGHT TO BE GLASSY IN NATURE AND FORMED AGGREGATES. PARTICLES WERE IN THE 5M-10M RANGE.Links
- Leatherhead Food International's searchable website with its research services and some market information
- USDA's “Dietary Guidelines for Americans 2005,” with estimated sources of sodium in food supply
- CDC's National Health and Nutrition Examination Survey (NHANES), 1999-2000, for actual and recommended intakes of selected nutrients
- For more information on reducing salt in formulations, use the key words “reduced salt” in the home page search field
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!


